Laboratory work №6: The combustion process
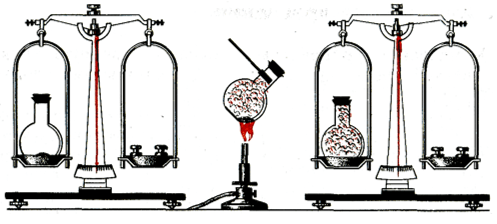
In this laboratory work you will learn about the combustion process Long-term plan section Subsections of the standard curriculum Learning objectives Air. Combustion reaction Air. The composition of the air. Laboratory experiment No. 6 “Candle Combustion” 7.3.1.1 know the composition of the air; 7.3.1.2 know that combustion process consumes oxygen, which is part of the air; 7.3.1.3 understand the importance of protecting atmospheric air from pollution
Laboratory work №6: The combustion process Read More »