Isotope stability and radioactive decay
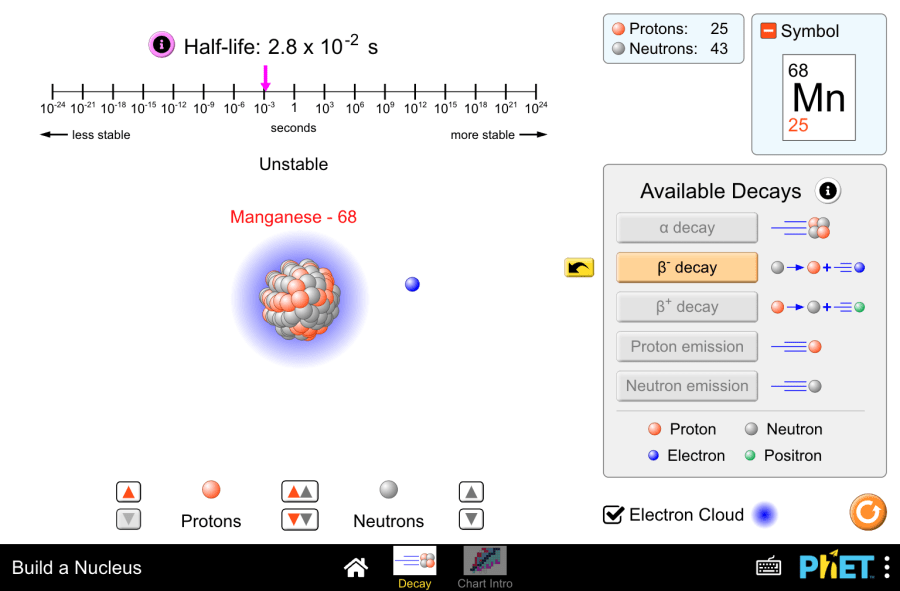
Build a Nucleus by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0 (https://phet.colorado.edu) The title of the Project: Isotope stability and radioactive decay This virtual laboratory is intended for use in chemistry classes on the following topics: Purpose of the virtual laboratory work: Characteristics of atomic particles Table 1 Particles Mass number Charge number Note proton 1 +1 The number of protons is equal to the ordinal number of the element neutron 1 0 The number of neutrons can be found by the following formula:N=А r -Z , А r -atomic mass number, Z – number of protons electron 0 -1 The number of electrons is equal to the ordinal number of the element Practical part 1.Launch the simulation. You will have two screens to choose: “Decay” and “Chart intro”. Let’s start with the “Decay” screen. 2.In this screen you can build a nuclide from protons and neutrons. It allows building of nuclides up to 94 protons and 146 neutrons.You can place neutrons and protons by dragging them into the play area or by using the spinners. The spinner between the protons and neutrons places both a proton and neutron into the nucleus simultaneously. 3.As the number of nucleons in the nucleus changes, the sim updates the element symbol (eg. Helium-5) number of protons and neutrons, and stability of the nuclide (stable or unstable) 4.“Info” button by half-life opens a static timeline with a dynamic nuclide half-life display. 5.Five decay types are represented within the simulation: α decay, β+ decay, β- decay, proton emission, and neutron emission. 6.Unstable nuclides decay into stable nuclides through one of these decay paths. Virtual experiment 7.Build an unstable isotope. For example, Beryllium-8. See the available decay. 8.Analyze the decay icon symbols to predict the final element after decay. Then press the button to see the animated decay process. 9.Observe how the various readouts (symbol, half-life, nucleon counter) update. Determine the resulting element. Write the balanced nuclear decay equation for the observed process. 10.Try another isotopes with different decays. 11.You can press the yellow button to watch the animation again. Determine the resulting element. Write the equation. Conclusion This virtual experiment effectively demonstrated radioactive decay using PhET’s “Build a Nucleus” simulation. By manipulating unstable isotopes, students visualized the decay process and explored the concept of half-life. This interactive exploration provides a valuable foundation for understanding the fascinating world of nuclear reactions.
Isotope stability and radioactive decay Read More »