Concentration
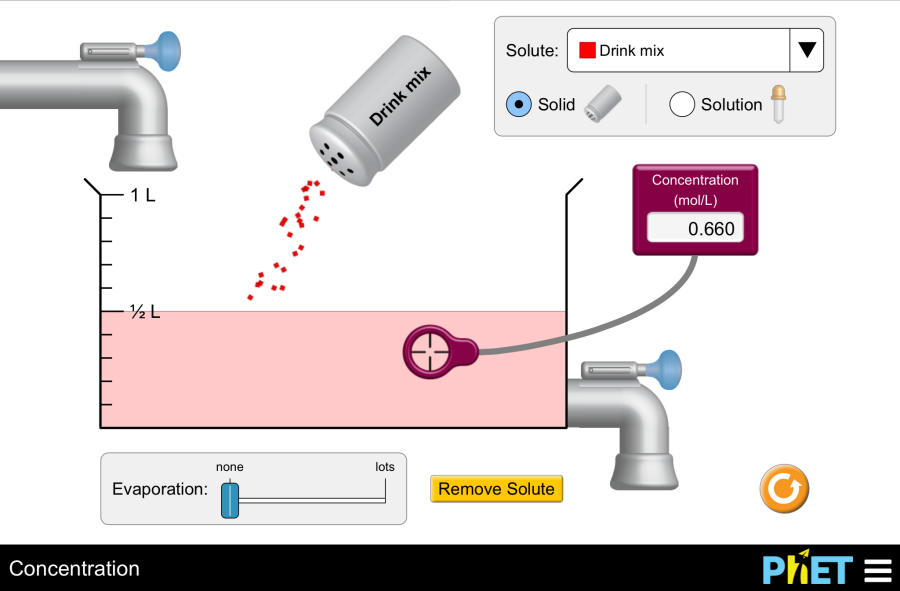
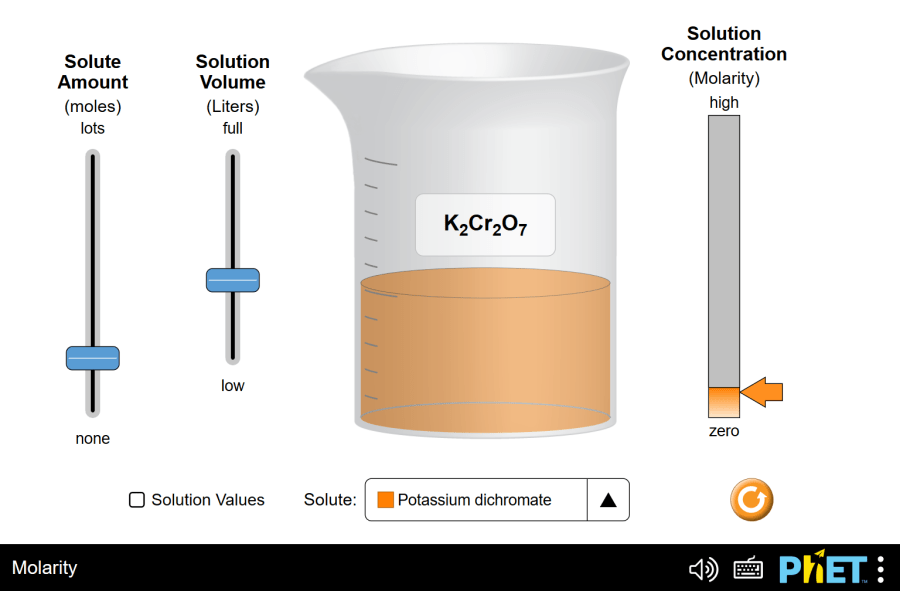
Concentration by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0 (https://phet.colorado.edu) The title of the Project: Concentration This virtual laboratory is intended for use in chemistry classes on the following topics: Objectives: Practical part This simulation helps you to identify the relationships between solution components and concentration. Virtual experiment No.1. 3. Let’s observe how potassium permanganate dissolves. Add the solute to the water. Notice the solution’s color change, indicating the presence of the solute. 4. Use the “Evaporation” slider to remove water from the beaker. What happens? Based on your observations, explain the term “saturated solution.” Virtual experiment No.2. Time to explore the connection between concentration and solution components. 5. Drag the concentration meter into the liquid. Observe how the concentration reading changes when you add solute, add water, or remove solution from the beaker. 6. Now, reset the simulation and choose a new solute, like nickel (II) chloride. This time, use the dropper to add the solute to the beaker. 7. Drag the concentration meter and place it below the dropper to measure the initial concentration before adding more solute. Record this number. 8. Use a formula to calculate the concentration of the diluted solution. C1 * V1 = C2 * V2 C2= (C1*V1)/V2 where: C1 = Initial concentration (known) V1 = Initial volume (assumed constant as no solute is added or removed before measurement) C2 = Final concentration (unknown, what they are trying to calculate) V2 = Final volume (known from the simulation) 9. Let’s check your prediction! Place the concentration meter back into the beaker to see the actual concentration in the simulation. Does it match your calculation? Repeat this experiment with different solutes to solidify your understanding of concentration changes. Make a conclusion. Conclusion By following these steps and encouraging exploration with various solutes, students can gain valuable insights into solution concentration using the PhET “Concentration” simulation.