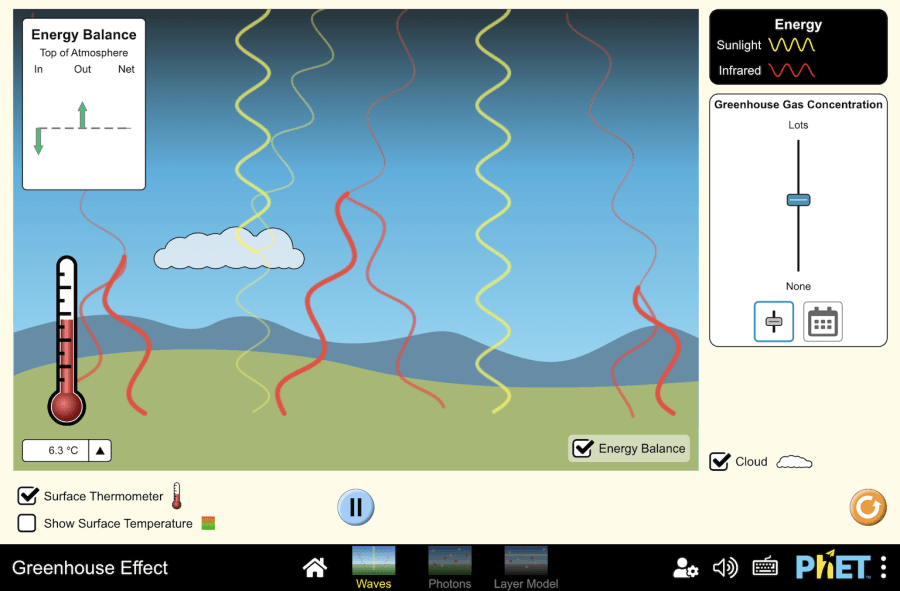
Greenhouse effect
Objective: Virtual experiment: Virtual simulation illustrates the driving forces of the greenhouse effect without describing the effects of climate change. The model demonstrates the basic concept of the greenhouse effect by considering only two types of radiation: solar (visible) and infrared. It is important to note that this model does not include the effects of actual greenhouse gases. The figure below shows the functions that each button performs. Workflow: Step 1. When you enter the Greenhouse Effect virtual experiment, you will see three sections: “Waves”, “Photons”, and “Layer Model”. As part of this activity, you will need to select the “Waves” section and perform a number of experiments in this section. First, open the simulation and run a sunlight simulation. Step 2. Activate the buttons to display the energy balance and surface temperature. Step 3. Observe the temperature change with the surface thermometer and how sunlight and infrared light enter and exit the ground with the energy balance. Step 4. Increase the concentration of greenhouse gases. Increasing the concentration of greenhouse gases increases the likelihood of infrared absorption. Keep an eye on the changes. Step 5. Decrease the concentration of greenhouse gases. Observe the changes. Step 6. At the bottom of the “greenhouse gas concentrations” section is a “calendar”. Click the button and open the calendar. You have the time of the ice age, 1750, 1950, 2020. Step 7. You can see the impact of the greenhouse effect in 1750. Step 8. Change the calendar to the ice age. Keep track of the changes. Step 9. Change the calendar to 1950. Keep track of the changes. Step 10. Move the calendar to the year 2020. Keep track of the changes. Conclusion The relationship between surface temperature and greenhouse gas concentrations has been described.